Enhancing Protein and Peptide Drugs with PEGylation
Time:2024-01-08 Hits:253
PEG modification has widespread applications in improving the performance of protein and peptide drugs. It involves covalently combining PEG with drugs to form complexes, offering benefits such as controlled drug release, extended half-life, reduced immunogenicity, lowered toxicity, enhanced drug targeting, and minimized dosage and administration frequency. PEGylation significantly enhances the pharmacokinetic and pharmacodynamic properties of various drugs.
Protein drugs, encompassing cytokines, enzymes, antibodies, and hormones, face challenges like short half-life, immunogenicity, low solubility, high toxicity, and susceptibility to degradation by proteases, thereby diminishing their clinical efficacy.
Peptide drugs, employed for disease prevention, treatment, and diagnosis, cover a range of types, including peptide vaccines, anti-tumor peptides, anti-viral peptides, peptide-directed drugs, cytokine mimetic peptides, antibacterial active peptides, and diagnostic peptides.
Mechanism of PEGylation
PEG modification of proteins and peptides extends drug half-life and reduces immunogenicity while preserving biological activity. The process involves chemically modifying the protein with PEG, causing an increase in molecular weight and a change in spatial structure. PEG acts as a protective barrier, preventing degradation by proteases and minimizing antibody production. Modified proteins, exceeding the glomerular filtration threshold, can evade filtration in the kidneys, resulting in prolonged blood circulation.
Main Modification Site
Polar amino acid residues, including cysteine, lysine, and terminal nitrogen acids, are common targets for PEG modification. The nucleophilic activity generally decreases in the order: thiol > α-amino > ɛ-amino > carboxyl > hydroxyl.
Reactions between PEG and proteins or peptides involve acylation, alkylation, redox reactions, aromatic ring substitution, and more. Key modification pathways include amino modification (N-terminal acylation, lysine side chain acylation, N-terminal alkylation), carboxyl modification, sulfhydryl modification, and other techniques such as pH control, metal ion or enzyme catalysis, and site-directed modification of glycosyl groups in glycoproteins.
1)Amino Modification
Commonly used amino modifiers include PEG-SS, PEG-SC, PEG-SPA, PEG-NHS, PEG-BTC, PEG-CHO, PEG-ALD, PEG-tresylate, and PEG-epoxide.
To investigate the effective modification of Human Leucocyte Antigen (HLA) using mPEG, a study involved the modification of lymphocyte surface HLA-I class antigen with mPEG-BTC. Results demonstrated a negative microlymphocytotoxicity test, indicating complete blocking of the specific immune response between HLA-I antigens and their corresponding antibodies after modification with mPEG [1].
2)Octreotide Modification:
Octreotide underwent chemical modification using SPA-mPEG (MW 2000) or ALD-mPEG (MW 2000/5000). The biological activity detection revealed that SPA-mPEG modification resulted in reduced activity, while ALD-mPEG, specifically modified at the N-terminus of octreotide, maintained biological activity. Furthermore, ALD-mPEG 5K significantly improved the pharmacokinetic properties [2].
3)Thiol Modification:
Sulfhydryl groups are present only in cysteine within protein-neutralizing peptides. PEG modifiers, leveraging the high nucleophilicity of sulfhydryl groups, can be selectively chosen for directional modification of proteins and peptides. For proteins lacking cysteine, sulfhydryl reaction sites can be introduced through genetic engineering or Traut’s reagent (2-imino hydrochlorothiol). Commonly used thiol modifiers include PEG-Mal, PEG-OPSS, PEG-VS, PEG-IA, PEG-DAQ, and PEG-Se. A case study involving the site-specific modification of human ciliary neuronotrophic factor (hCNTF) using mPEG-MAL (MW 40kDa) demonstrated a modification rate exceeding 90%, resulting in a 95% purity single modification product. This led to a remarkable 30.3 times increase in in vivo circulating half-life compared to the original protein [3].
4)Carboxyl Modification:
Carboxyl modification sites include aspartic acid, glutamic acid, and terminal carboxyl groups. The activation of the carboxyl group involves converting PEG's carboxyl group into an amino group, followed by combination with the protein's carboxyl group in the presence of DCCI or EDC. Commonly used carboxyl modifiers are PEG-HZ or PEG-NH2 [4].
While the terminal ends of single-chain polyethylene glycol have limitations in modifying new conjugated drugs, leading to low drug loading capacity, advancements in branched polyethylene glycol offer diverse conjugation possibilities. Branched structures, such as branched PEG, forked PEG, and multi-arm PEG with a dendritic structure at the end, significantly enhance drug loading capacity and provide varied conjugation properties.
PEGylation Agents List
|
货号
|
品名
|
描述
|
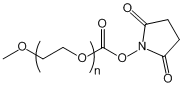
| ECS4133A | mPEG-NHS, MW 350 |
mPEG-Succinimidyl ester
|
| AJL0190A | mPEG-SC, MW 1K | |
| ACS0112B | mPEG-SC, MW 2K | |
| ECS4382A | mPEG-SC, MW 3.4K | |
| AJL0190B | mPEG-SC, MW 5K | |
| ACS0115B | mPEG-SC, MW 10K | |
| ECS4219A | mPEG-SPA, MW 1K |
mPEG-Succinimidyl Propionate
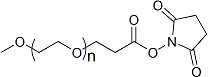
|
| ECS4356A | mPEG-SPA, MW 2K | |
| ECS4275A | mPEG-SPA, MW 5K | |
| ECS4094A | mPEG-SPA, MW 20K | |
| ECS4284A | mPEG-SS, MW 5K |
mPEG-Succinimidyl Succinate ester
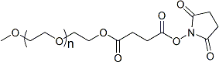
|
| ECS2001A | mPEG-SCM, MW 20K |
mPEG-Succinimidyl Carboxymethyl Ester
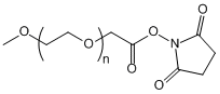
|
| ECS2103A | mPEG-SCM, MW 40K | |
| ECS4171A | mPEG-SG, MW 2K |
mPEG-Succinimidyl Glutarate ester
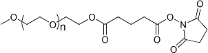
|
| ECS4334A | mPEG-SVA, MW 5K |
mPEG-Succinimidyl valerate
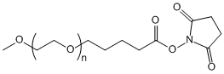
|
| ECS4154A | mPEG-Mal, MW 350 |
mPEG-Maleimide
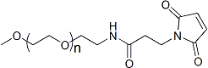
|
| ECS4155A | mPEG-Mal, MW 500 | |
| ECS4156A | mPEG-Mal, MW 1K | |
| DCS0133A | mPEG-Mal, MW 2K | |
| ECS4168A | mPEG-Mal, MW 4K | |
| ACS0135B | mPEG-Mal, MW 5K | |
| ACS0136A | mPEG-Mal, MW 10K | |
| ACS0137A | mPEG-Mal, MW 20K | |
| ECS4150A | mPEG-ALD, MW 1K |
mPEG-Aldehyde

|
| ECS4194A | mPEG-CHO, MW 2K | |
| ACS0121A | mPEG-CHO, MW 5K | |
| ECS4212A | mPEG-EPO, MW 550 |
mPEG-Epoxide
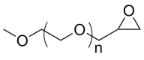
|
| ECS4198A | mPEG-EPO, MW 2K | |
| ECS4182A | mPEG-EPO, MW 10K | |
| ECS4317A | mPEG-HZ, MW 350 |
mPEG-Hydrazide
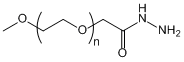
|
| ECS4278A | mPEG-HZ, MW 2K | |
| ECS4157A | mPEG-NH2, MW 350 |
mPEG-Amine
|
| ECS4244A | mPEG-NH2, MW 550 | |
| ECS2165A | mPEG-NH2, MW 1K | |
| ACS0105B | mPEG-NH2, MW 2K | |
| ACS0106B | mPEG-NH2, MW 3.4K | |
| ECS4296A | mPEG-NH2, MW 4K | |
| ACS0107B | mPEG-NH2, MW 5K | |
| ECS2180A | mPEG-NH2, MW 10K | |
| ACS0109B | mPEG-NH2, MW 20K | |
| ECS2069C | Mal-PEG-SCM, MW 2K |
Maleimide-PEG-Succinimidyl Carboxymethyl Ester
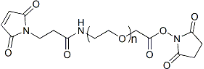
|
| ECS2069B | Mal-PEG-SCM, MW 5K | |
| ECS2069A | Mal-PEG-SCM, MW 10K | |
| ECS4208A | Mal-PEG-NHS, MW 200 |
Maleimide-PEG-NHS
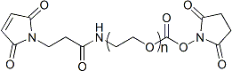
|
| ACS1695B | Mal-PEG-NHS, MW 2K | |
| ECS2072A | Mal-PEG-NHS, MW 3.4K | |
| ECS2065A | Mal-PEG-NHS, MW 5K | |
| ECS4137A | MAL-PEG-SVA, MW 5K |
Maleimide-PEG-Succinimidyl valerate
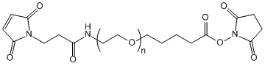
|
| ECS4192A | Mal-PEG-HZ, MW 2K |
Maleimide-PEG-Hydrazide
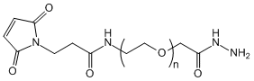
|
| ECS2005A | NHS-PEG-SH,MW 200 |
Thiol-PEG- Succinimidyl ester
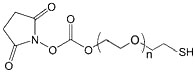
|
| ECS2006A | NHS-PEG-SH,MW 400 | |
| ECS2007A | NHS-PEG-SH,MW 500 | |
| ECS2017A | SH-PEG-NHS, MW 600 | |
| ECS2009A | NHS-PEG-SH,MW 800 | |
| ECS2010A | NHS-PEG-SH,MW 1K | |
| ECS4307A | SH-PEG-NHS, MW 2K | |
| ECS4407A | SH-PEG-NHS, MW 5K | |
| ECS4205A | SH-PEG-NH2, MW 600 |
Thiol-PEG-Amine
|
| ECS4095A | SH-PEG-NH2, MW 2K | |
| ECS1978B | SH-PEG-NH2, MW 3.4K | |
| DCS2049A | SH-PEG-NH2, MW 5K | |
| ECS4142A | SH-PEG-NH2, MW 10K | |
| ECS2096A | SH-PEG-COOH, MW 400 |
Thiol-PEG-Acid
|
| ECS4245A | SH-PEG-COOH, MW 1K | |
| ECS1991B | SH-PEG-COOH, MW 2K | |
| ECS2021A | SH-PEG-COOH, MW 3.4K | |
| ECS2118A | SH-PEG-COOH, MW 5K | |
| ECS2182A | SH-PEG-CHO, MW 5K |
Thiol-PEG- Aldehyde
|
| ECS4174A | SH-PEG-SH, MW 400 |
Thiol-PEG-Thiol
|
| ECS4173A | SH-PEG-SH, MW 600 | |
| ECS2084A | SH-PEG-SH, MW 2K | |
| ECS4143A | SH-PEG-HZ, MW 10K |
Thiol-PEG-Hydrazide
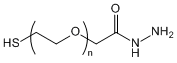
|
| ECS4383A | SH-PEG-Biotin, MW 200 |
Thiol-PEG-Biotin
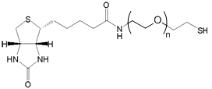
|
| ECS4412A | SH-PEG-Biotin, MW 5K | |
| ECS4292A | SC-PEG-SC, MW 400 |
Succinimidyl ester-PEG-Succinimidyl ester
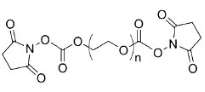
|
| ECS2117B | SC-PEG-SC, MW 2K | |
| ECS4233A | SC-PEG-SC, MW 3.4K | |
| ECS4264A | SC-PEG-SC, MW 5K | |
| ECS4311A | SC-PEG-SC, MW 10K | |
| ECS4255A | NHS-PEG-COOH, MW 3.4K |
NHS-PEG-Acid
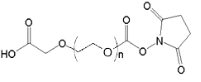
|
| ECS2071A | NHS-PEG-VS, MW 3.4K |
NHS-PEG-Vinylsulfone
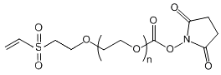
|
| ECS4135A | NHS-PEG-OH, MW 2K |
NHS-PEG-Hydroxy
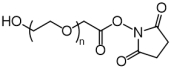
|
| ECS4354A | SS-PEG-SS, MW 2K |
Succinimidyl Succinate ester-PEG-Succinimidyl Succinate ester
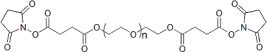
|
| ECS4172A | SG-PEG-SG, MW 2K |
Succinimidyl Glutarate ester-PEG-Succinimidyl Glutarate ester
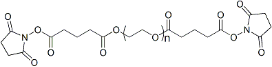
|
| ECS4231A | SG-PEG-SG, MW 3.4K | |
| ECS2129A | SPA-PEG-SPA, MW 3.4K |
Succinimidyl Propionate-PEG-Succinimidyl Propionate
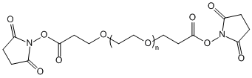
|
| ECS4290A | SCM-PEG-SCM, MW 2K |
Succinimidyl Carboxymethyl Ester-PEG-Succinimidyl Carboxymethyl Ester
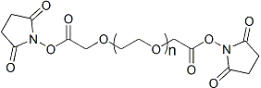
|
| ECS4195A | SCM-PEG-SCM, MW 5K | |
| ECS4291A | SCM-PEG-SCM, MW 20K | |
| ECS4298A | OPSS-PEG-NHS, MW 5K |
Ortho-Pyridyldisulfide-PEG-NHS
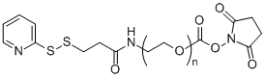
|
| ECS4082A | OPSS-PEG-SCM, MW 5K |
Ortho-Pyridyldisulfide-PEG-Succinimidyl Carboxymethyl Ester
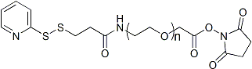
|
| ECS4199A | AC-PEG-SC, MW 2K |
Acrylate-PEG-NHS
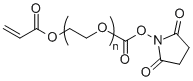
|
| ECS2051A | AC -PEG-SVA, MW 3.4K |
Acrylate-PEG-Succinimidyl valerate
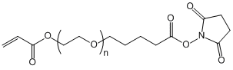
|
| ECS2098A | AC-PEG-SCM, MW 2K |
Acrylate-PEG-SCM
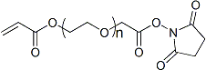
|
| ECS4247A | Biotin-PEG-SCM, MW 600 |
Biotin-PEG-Succinimidyl Carboxymethyl Ester
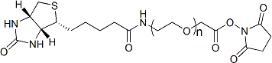
|
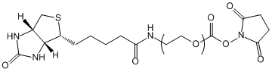
| ECS4343A | Biotin-PEG-SC, MW 2K |
Biotin-PEG- Succinimidyl ester
|
| EYD0528B | Biotin-PEG-SC, MW 5K | |
| ECS4248A | Biotin-PEG-SS, MW 600 |
Biotin-PEG-Succinimidyl Succinate ester

|
| ECS4249A | Biotin-PEG-SG, MW 600 |
Biotin-PEG-Succinimidyl Glutarate ester

|
| ECS4246A | Biotin-PEG-SVA, MW 600 |
Biotin-PEG-Succinimidyl valerate
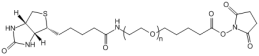
|
| ECS4162A | CHO-PEG-OH, MW 2K |
Aldehyde-PEG-Hydroxy
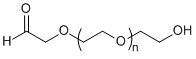
|
| ECS2174A | CHO-PEG-CHO, MW 2K |
Aldehyde-PEG-Aldehyde
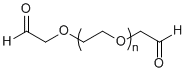
|
References:
[1] Dong Hee Na, Kang Choon Lee, Patrick P DeLuca. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharm Res, 2005, 22(5): 743-9.
[2] Kynclova E, Elsner E, K?pf A, et al. Novel method for coupling of poly(ethyleneglycol) to carboxylic acid moieties of proteins. Journal of Molecular Recognition, 1996, 9(5-6):644-651.
