Helicobacter pylori Antigen (HP) - Immunochromatography (Colloidal Gold and Colored Latex) Solution
Time:2024-03-07 Hits:182
Project Background
From "Guiding Principles for Technical Review of Registration of Helicobacter Pylori Antigen/Antibody Detection Reagents"
Helicobacter pylori (HP) is a Gram-negative microaerophilic bacterium that resides in the stomach and duodenum. Its infection is highly prevalent, with a global natural population infection rate exceeding 50%. Factors influencing the infection rate of Helicobacter pylori include economic status, living conditions, education level, occupation, and drinking habits. Generally, developing countries exhibit higher rates than developed countries. Currently, it is understood that humans are the sole source of infection for Helicobacter pylori in the natural environment, and oral infection is presumed to be the primary transmission route.
Histologically, almost all Helicobacter pylori-infected patients experience a chronic active inflammatory reaction. The latest expert consensus defines HP gastritis as an "infectious (infectious) disease," yet over 70% of infected patients show no symptoms. For others, the infection can lead to chronic gastritis, peptic ulcers, and more. Common symptoms include upper stomach discomfort, flatulence, anorexia, nausea, vomiting, and dark or tar-colored stools. Research indicates that Helicobacter pylori is the primary causative factor for gastritis and peptic ulcers, and it is closely linked to functional dyspepsia, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. The World Health Organization's International Agency for Research on Cancer has classified it as a carcinogen. International guidelines on Helicobacter pylori infection emphasize that eradicating the bacterium can significantly reduce the incidence of gastric and duodenal diseases, including gastric cancer, and may reduce new cases of Helicobacter pylori infection in the future.
Diagnostic methods for Helicobacter pylori infection are categorized into invasive and non-invasive methods based on the material being examined. Invasive methods rely on gastroscopy and include histological testing (e.g., HE staining, Warthin-Starry silver staining, modified Giemsa staining, toluidine blue staining, acridine orange staining, immunohistochemical staining), bacterial culture, and the rapid Urease test (RUT). Non-invasive methods encompass serology (antibody) testing, fecal antigen testing, and the urea breath test (UBT). Each diagnostic method has its own advantages and limitations.
The latest international and domestic expert consensus on the diagnosis of HP infection is as follows:
a. For diagnosing HP infection (in patients not treated with antibiotics), ① UBT is the most recommended method, and (monoclonal antibody) stool antigen testing can also be applied; ② If the subject has indications for endoscopy but no contraindications for biopsy, the RUT test is recommended; for gastritis biopsy specimens, histological staining can diagnose HP infection, and immunohistochemical staining or other special staining can be used for negative cases; ③ HP culture is only performed when drug susceptibility testing is considered; ④ Serological antibody detection can be used in conditions such as gastrointestinal bleeding, severe atrophic gastritis, gastric MALT lymphoma, gastric cancer, and other special cases.
b. For evaluating HP eradication effectiveness, UBT is the preferred method, with (monoclonal antibody) stool antigen testing as an alternative; serum antibody testing is not applicable.
The Helicobacter pylori antigen detection reagent described in this article refers to a reagent that uses colloidal gold immunochromatography or colored latex immunochromatography based on the principle of the antigen-antibody reaction to perform in vitro qualitative detection of Helicobacter pylori antigen in human stool samples. When combined with clinical manifestations and other laboratory indicators, it can assist in diagnosing Helicobacter pylori infection in the population. Fecal antigen detection can also be utilized to evaluate the eradication effect of Helicobacter pylori.
Performance Evaluation
Colloidal Gold Immunochromatography Platform:
Antibody Pairing Combination: HP Helicobacter pylori antibody (Product No.: EKY0913D) labeled colloidal gold (sprayed on the release pad) + HP Helicobacter pylori antibody (Product No.: EKY0913E) for streaking;
Dilution of HP Positive Bacteria Liquid: P1 (Cat. No.: EEI0276A), P2 (Cat. No.: EEI0275A), P5 (Cat. No.: EEI0279A) are diluted 1000 times with an appropriate diluent (0.9% NaCl solution is used this time), P3 (Cat. No.: EEI0277A), P4 (Cat. No.: EEI0278A) are diluted 10 times with a suitable diluent (0.9% NaCl solution is used this time).
The sample volume is 80ul, and the reaction time is 15 to 20 minutes;
Experimental Results: On the colloidal gold immunochromatography platform, the quality control of P1-P5 after dilution using this antibody pairing combination test was all positive, indicating that the sensitivity of this antibody pairing test was good.
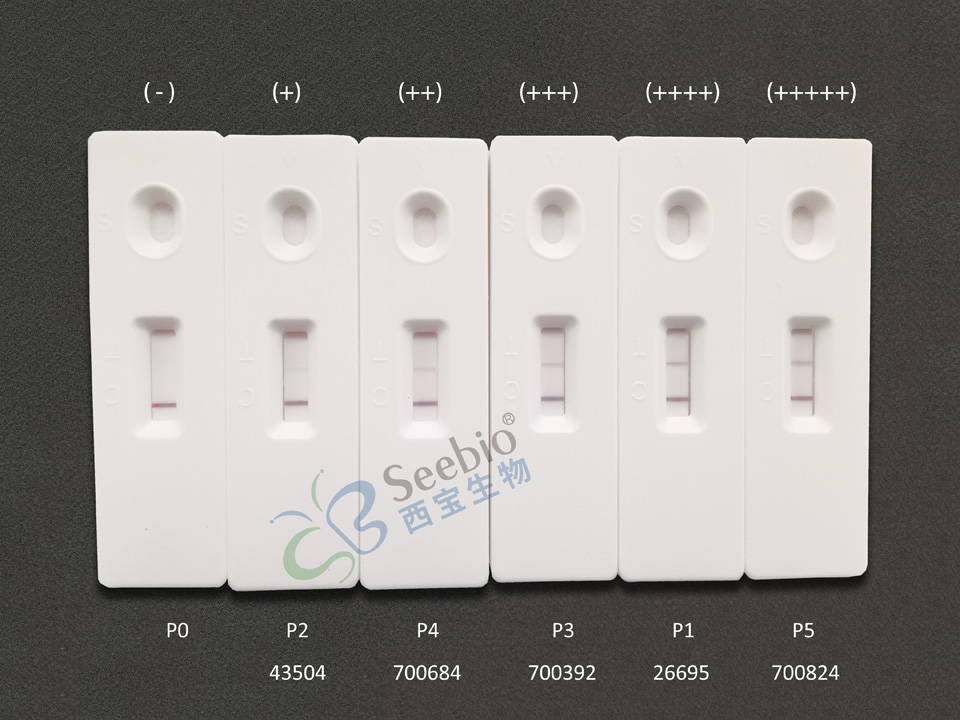
Schematic Diagram of Colloidal Gold Chromatography Platform Testing
Antibody Pairing Combination: HP Helicobacter pylori antibody (Cat. No.: EKY0913E) labeled with colored latex (sprayed on the release pad) + HP Helicobacter pylori antibody (Cat. No.: EKY0913D) for scratching;
Dilution of HP Positive Bacteria Liquid: P1 (Cat. No.: EEI0276A), P2 (Cat. No.: EEI0275A), P5 (Cat. No.: EEI0279A) are diluted 1000 times with an appropriate diluent (0.9% NaCl solution is used this time), P3 (Cat. No.: EEI0277A), P4 (Cat. No.: EEI0278A) are diluted 10 times with a suitable diluent (0.9% NaCl solution is used this time).
The sample volume is 80ul, and the reaction time is 15 to 20 minutes;
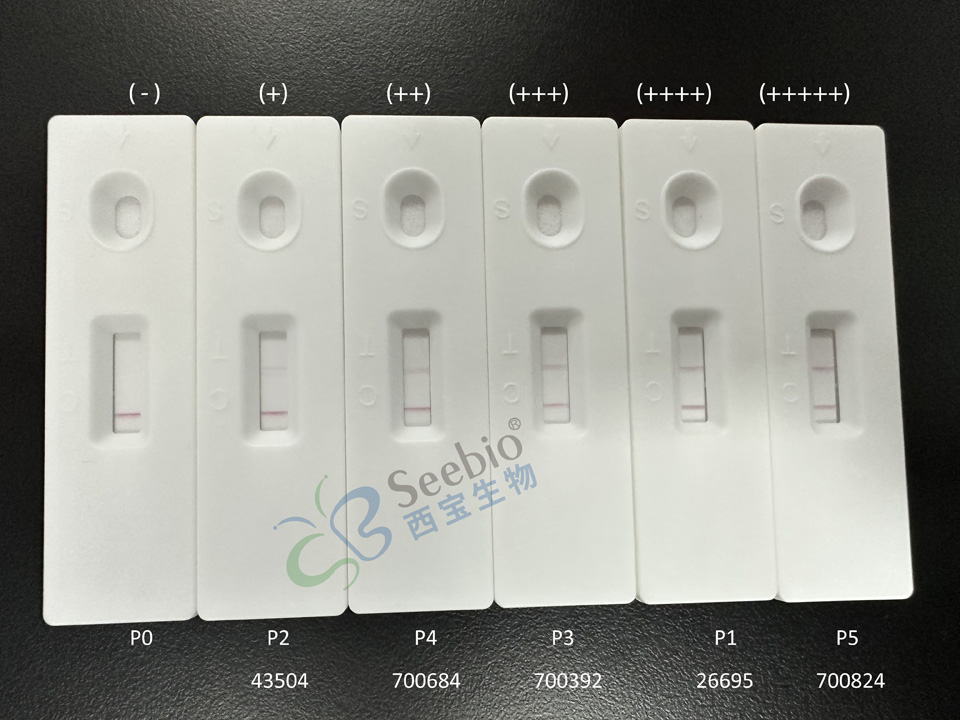
Experimental Results: On the color latex immunochromatography platform, the quality control of P1-P5 after dilution using this antibody pairing combination test was all positive, indicating that the sensitivity of this antibody pairing test was good.
For more product details, please contact us: service@seebio.cn or Phone: +86 21 58183719 or Wechat: +86 158 0195 7578
Product Information
|
Product name
|
Item number
|
Recommended pairing
|
|
HP Helicobacter pylori antibodies
|
EKY0913D
|
HP Helicobacter pylori antibody (Cat. No.: EKY0913D) labeled colloidal gold + HP Helicobacter pylori antibody (Cat. No.: EKY0913E) was streaked (Colloidal gold chromatography pair combination)
HP Helicobacter pylori antibody (Cat. No.: EKY0913E) labeled colored latex + HP Helicobacter pylori antibody (Cat. No.: EKY0913D) streak (Color latex chromatography pairing combination)
|
|
HP Helicobacter pylori antibodies
|
EKY0913E
|
|
|
HP Helicobacter pylori antibody (Cat. No.: EKY0913D)-Colloidal Gold Concentrate
|
DKY1316A
|
|
|
HP Helicobacter pylori antibody (Cat. No.: EKY0913E)-Colored Latex Concentrate
|
DKY1316B
|
|
|
Colloidal solution (40nm, 1OD)
|
DXJ9917B-100ml
|
|
|
Red carboxyl latex microspheres, 400nm
|
DEG0809A-1ml
|
|
|
Helicobacter pylori (HP) quality control products
HP Control26695(P1)
|
|
|
|
Helicobacter pylori (HP) quality control products
HP Control 43504(P2)
|
|
|
|
Helicobacter pylori (HP) quality control products
HP Control 700392(P3)
|
|
|
|
Helicobacter pylori (HP) quality control products
HP Control 700684(P4)
|
|
|
|
Helicobacter pylori (HP) quality control products
HP Control700824 (P5)
|
|
|
|
Other related paired reagents (double antibody sandwich method)
|
||
|
HP Helicobacter pylori antibodies
|
|
|
|
HP Helicobacter pylori antibodies
|
|
|
|
HP Helicobacter pylori antibodies
|
|
|
|
HP Helicobacter pylori antibodies
|
|
|
|
HP Helicobacter pylori IgG/IgM detection related reagents
|
||
|
Helicobacter pylori (HSP60) recombinant antigen
|
coated antigen
|
|
|
Helicobacter pylori (HP.UreB) recombinant antigen
|
coated antigen
|
|
|
Helicobacter pylori (HP.VacA) recombinant antigen
|
coated antigen
|
|
|
Helicobacter pylori (HP.CagA) recombinant antigen
|
coated antigen
|
|
|
Mouse anti-human IgG antibody
|
|
|
|
Mouse anti-human IgM antibody
|
|
|